
Metal roofing is known for its strength, longevity, and clean appearance, but even the most durable materials can deteriorate when corrosion begins to form beneath the surface.
One of the most common and often overlooked causes of roof rust is galvanic corrosion, an electrochemical reaction that occurs when two dissimilar metals come into contact in the presence of moisture. Over time, this reaction can cause fasteners, flashings, and panels to weaken, reducing the overall lifespan of the roof.
This article will cover what galvanic corrosion is, how it develops in roofing systems, which materials are most at risk, and the most effective ways to prevent it before serious damage occurs.
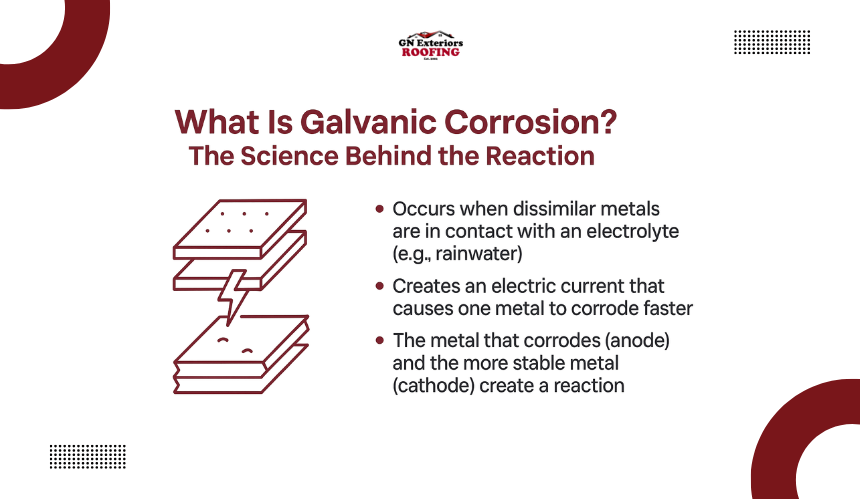
Galvanic corrosion occurs when two different metals come into electrical contact in the presence of an electrolyte, such as rainwater or condensation. This reaction creates a small electric current that causes one of the metals to corrode faster than it normally would.
The metal that deteriorates is known as the anode, while the more resistant one is called the cathode. This natural electrochemical process is what makes rust spread quickly across metal surfaces in mixed-metal roofing systems.
When used together without insulation or coating, certain metal combinations can accelerate this corrosion process. For example, when aluminum comes into contact with copper or steel, the softer metal (aluminum) often becomes the sacrificial element.
Moisture acts as a conductive path, allowing the corrosion to continue as long as the metals stay connected and the electrolyte remains present.
Galvanic corrosion starts with three essential components: two dissimilar metals, an electrolyte, and electrical contact. When these conditions align, electrons move from the more active metal (the anode) to the less active one (the cathode), creating a steady flow of energy. This reaction weakens the anode, causing visible rust or pitting over time.
Not all metal roofing materials react the same way when exposed to galvanic corrosion. The level of risk depends on the type of metal, its placement in the galvanic series, and how it interacts with other materials in the roofing system. Some metals corrode faster because they are more reactive, while others, like stainless steel or copper, are more stable and resist oxidation.
However, when incompatible metals come into contact, even the strongest materials can degrade under the right conditions.
Did you know? Up to 60% of premature metal roof failures in humid or coastal areas are traceable to galvanic activity between dissimilar materials such as copper and galvanized steel. This makes material compatibility one of the most critical factors in preventing roof corrosion. |
Mixing certain metals without insulation or coatings can lead to accelerated corrosion. Common high-risk combinations include:
These pairings should always be avoided or separated using non-conductive materials to slow the electrochemical reaction.
Did you know? Around 35–45% of all roof corrosion cases reported in mixed‑metal roofing systems are linked to galvanic reactions, mainly occurring at fasteners, flashing, and gutter joints. |
Fasteners and flashings are common starting points for corrosion because they directly connect different metals. Using incompatible nails or screws on metal roofing can create corrosion spots that expand over time.
For instance, stainless steel screws on aluminum panels may seem durable but can cause pitting around the connection points. Similarly, copper flashing near steel gutters can trigger localized rusting.
Choosing hardware made from the same or compatible materials as the roofing panels is crucial to maintaining integrity.
Environmental conditions significantly affect how fast galvanic corrosion progresses. Roofs in coastal regions face higher risks due to salt particles that act as electrolytes.
Urban areas with high pollution levels also accelerate metal oxidation, while humid or rainy climates keep surfaces damp for extended periods, allowing the reaction to continue.
Roofs in dry, inland regions tend to experience less galvanic activity but can still corrode if water pooling or trapped debris occurs.
Fact: Homes in coastal regions face corrosion rates 2–3 times higher than inland properties due to the conductivity of airborne salt particles. |
Galvanic corrosion often develops quietly, and by the time visible rust appears, damage may already have progressed beneath the surface. Early detection plays a key role in preventing costly repairs and extending the lifespan of a metal roof.
Homeowners and maintenance professionals should look for subtle but consistent patterns of wear that signal an active electrochemical reaction between dissimilar metals.
Moisture trapped near fasteners, junctions, or seams often provides the ideal environment for corrosion to begin. Even when the roof appears structurally sound, a closer inspection can reveal early warning indicators that warrant immediate attention.
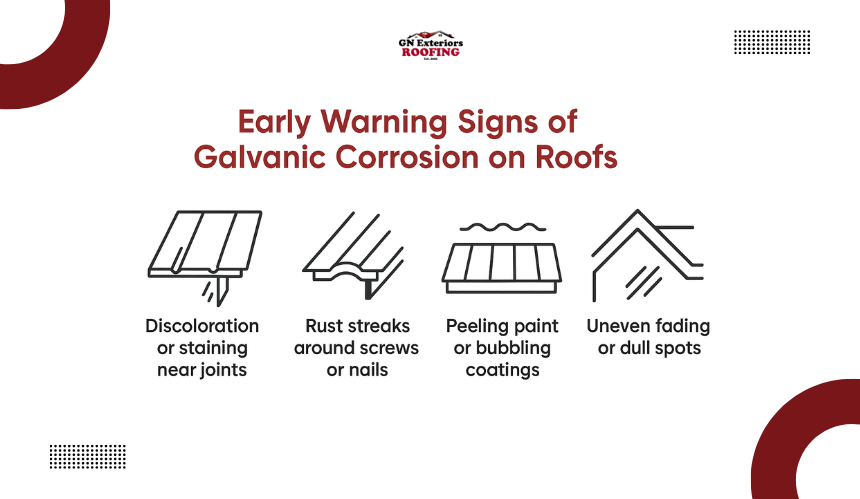
One of the first signs of galvanic corrosion is a change in surface appearance. Look for:
These signs often appear first on roofs with exposed fasteners or mixed-metal components that have not been properly isolated.
Not all corrosion is immediately visible. As the reaction progresses, small mechanical and structural issues may arise, such as:
These indicators show that the corrosion process is already affecting the roof’s stability, even if surface rust has not fully developed.
Professional inspection should be scheduled as soon as any corrosion-related symptom is identified. A qualified roofing expert uses tools like moisture meters, surface analyzers, and galvanic potential testers to detect early electrochemical activity before it spreads.
Regular inspections by a metal roofing expert every 12 to 18 months, especially in coastal or high-humidity regions, help identify risks before they escalate.

Preventing galvanic corrosion starts with smart design choices and consistent maintenance. The key is to minimize contact between dissimilar metals and reduce moisture exposure, which fuels the corrosion process. Below are some proven tips to prevent galvanic corrosion on roofs:
Select metals that are close in the galvanic series to reduce the chance of electrochemical reactions. Avoid pairing reactive metals like aluminum with copper or stainless steel.
Install non-conductive materials between metals to stop direct contact.
Protect exposed surfaces with anti-corrosion coatings such as zinc primers or epoxy-based paints. Recoat regularly, especially in coastal or high-moisture environments.
Routine care keeps corrosion from progressing unnoticed.
Consistent attention to these small details greatly reduces the risk of galvanic corrosion and extends the lifespan of any metal roofing system.
When galvanic corrosion appears on a roof, taking fast and proper action can prevent the issue from spreading. Repairs should target both the visible rust and the hidden electrochemical activity that weakens the metal from within. Knowing how to identify, treat, and restore affected areas ensures the roof remains structurally sound and weather-resistant.
Before repairs begin, it’s essential to determine how deeply the corrosion has affected the roofing components.
Roofing professionals use several effective techniques to stop corrosion and restore the roof’s integrity:
Small, surface-level rust patches can sometimes be handled with simple DIY fixes like sanding, priming, and resealing. However, larger or spreading corrosion needs expert care.
Professionals can accurately test for hidden corrosion, apply industrial-grade coatings, and ensure all materials are compatible to prevent the issue from returning.
If you notice signs of corrosion or rust on your metal roof, don’t wait for the damage to worsen. GN Exteriors provides expert inspection, repair, and corrosion treatment services designed to restore your roof’s strength and longevity. Our team ensures that every component is properly protected and built to withstand years of exposure to moisture and weather.
Yes. When solar panels with aluminum frames are mounted on roofs using steel or copper brackets, galvanic corrosion can occur at contact points. Using insulated mounts and non-conductive spacers prevents the reaction and protects both the panels and roofing materials.
The timeframe varies depending on climate and material pairing. In coastal or humid regions, visible corrosion can appear within a year, while in dry environments, it may take several years to become noticeable. Regular inspections help catch early signs before structural damage develops.
Yes. Professionals use tools such as galvanic potential meters and coating thickness gauges to measure voltage differences between metals and identify weak spots where corrosion is likely to start. These diagnostic methods help in planning timely maintenance.
It does. When water flows from a more noble metal, like copper, onto a less noble one, such as steel or aluminum, it can carry ions that accelerate corrosion on the receiving surface. Proper roof design should ensure that runoff paths are isolated or directed away from vulnerable materials.
Protective coatings significantly reduce the risk but don’t eliminate it entirely. Over time, coatings can wear down, exposing the metal beneath. Regular reapplication and visual checks are essential for long-term protection.